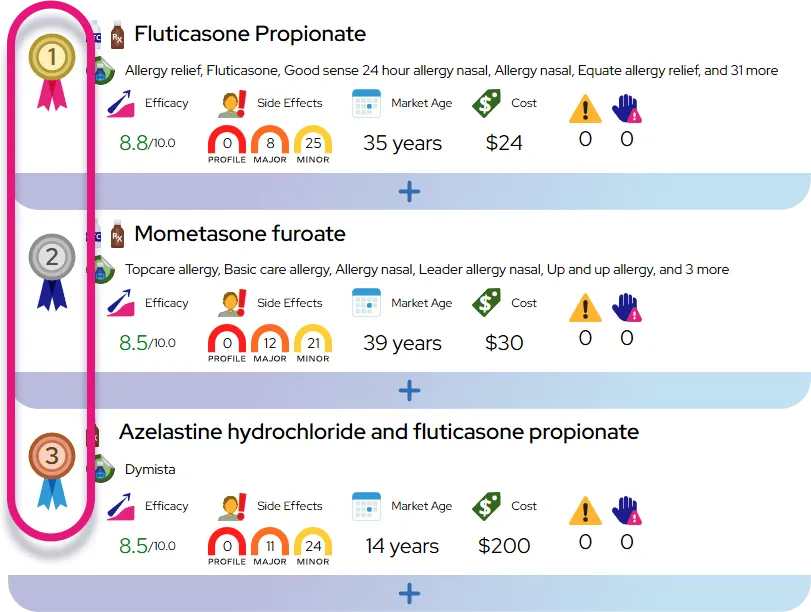
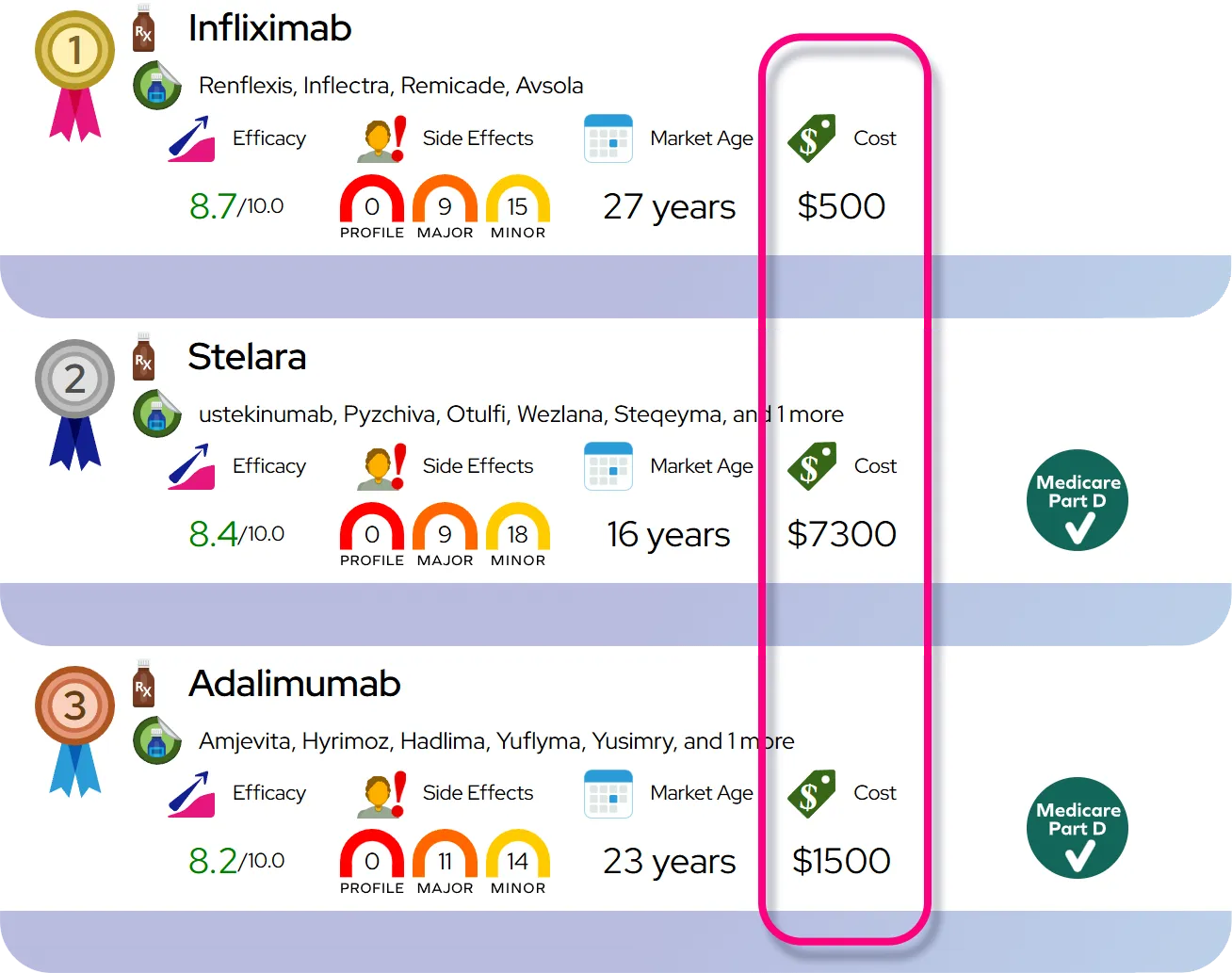
At a Glance
How It Works
Lisdexamfetamine is a "prodrug," meaning it is inactive until your body converts it into dextroamphetamine, a stimulant that works mainly in the brain.- After swallowing, enzymes in the blood slowly change lisdexamfetamine into dextroamphetamine, which helps provide a smoother, longer‑lasting effect.
- Dextroamphetamine increases levels of natural chemicals like dopamine and norepinephrine that support attention, impulse control, and alertness.
- These changes can reduce ADHD symptoms and, in binge eating disorder, can help decrease urges to binge and the number of binge episodes.
Treatment and Efficacy
Approved indications
Lisdexamfetamine dimesylate is approved for:
- Attention‑deficit/hyperactivity disorder (ADHD) in children 6–17 years and adults.
- Moderate to severe binge eating disorder (BED) in adults (not for weight loss or obesity treatment).
Common off‑label uses (evidence varies)
Clinicians may sometimes use lisdexamfetamine off label for conditions such as narcolepsy or as an add‑on in certain treatment‑resistant depressive or fatigue states, but evidence is more limited than for approved uses and other medications are often preferred first line.
Efficacy in ADHD
Many people notice some improvement in attention, hyperactivity, and impulsivity on the first day or within several days, with full benefit often assessed over 1–4 weeks as the dose is adjusted.
In clinical trials, lisdexamfetamine produced large reductions in ADHD symptom scores and meaningful improvements in school, work, and home functioning, comparable overall to other long‑acting stimulant medications.
Efficacy in binge eating disorder
For BED, reductions in the number of weekly binge days often begin within the first week or two, with many patients experiencing substantially fewer or no binge episodes at effective doses (typically 50–70 mg/day).
Comparison to similar drugs
Effectiveness is generally similar to other amphetamine and methylphenidate stimulants, but as a prodrug it tends to provide a smoother onset and offset and may have slightly lower abuse potential, while still carrying the same Schedule II controlled‑substance risks.
Dosage and Administration
General dosing principles
Lisdexamfetamine is taken by mouth once daily in the morning, with or without food, using capsules or chewable tablets; doses are individualized and increased gradually based on benefit and tolerability.
Typical ADHD dosing
For children 6–17 years and adults with ADHD, a common starting dose is 30 mg once each morning, increasing by 10–20 mg at roughly weekly intervals to a usual range of 30–70 mg/day (maximum 70 mg/day).
Typical binge eating disorder dosing (adults)
For moderate to severe BED, adults often start at 30 mg once daily and increase to 50–70 mg/day (maximum 70 mg/day) as tolerated and needed to reduce binge episodes.
How to take the medicine
Capsules may be swallowed whole or opened and the entire contents mixed with water, yogurt, or orange juice and consumed right away without chewing the granules; chewable tablets should be chewed completely before swallowing and not divided into partial doses.
Because it can cause insomnia, it is usually taken early in the morning and avoided in the afternoon or evening.
Special dosing instructions
In severe kidney impairment, the maximum recommended daily dose is reduced (for example, to 50 mg/day in severe impairment and 30 mg/day in end‑stage renal disease), and careful monitoring is needed.
Clinicians may occasionally recommend brief dose interruptions (for example, to reassess ongoing need or manage side effects), but any changes should be supervised rather than done abruptly on one’s own.
Missed dose
If a dose is missed, it is usually taken as soon as remembered that same morning; if it is later in the day or close to the next dose, the missed dose should be skipped and the regular schedule resumed the next day.
Do not take extra doses or double up to make up for a missed dose.
Overdose
Suspected overdose (such as extremely fast heartbeat, severe agitation, hallucinations, chest pain, or loss of consciousness) is a medical emergency; seek emergency care or call poison control at 1‑800‑222‑1222 in the United States immediately.
Safety and Side Effects
Common side effects
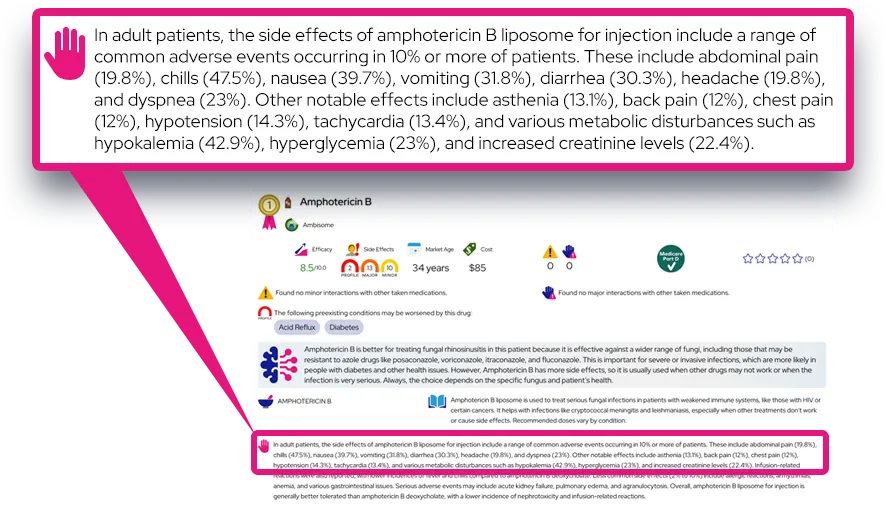
Common effects include decreased appetite, weight loss, dry mouth, trouble sleeping, increased heart rate, increased blood pressure, irritability, anxiety, nausea, stomach pain, and headache.
These usually start soon after treatment begins or after a dose increase, are often mild to moderate, and may lessen over time; appetite and sleep changes are among the most frequent reasons for dose adjustments.
Serious or rare adverse effects (seek immediate care)
Serious reactions can include chest pain, fainting, shortness of breath, or other signs of heart problems; sudden severe headache, weakness, or trouble speaking; new or worsening behavior changes, aggression, hallucinations, or manic symptoms; seizures; signs of serotonin syndrome (high fever, confusion, stiff muscles, fast heartbeat, sweating); painful or prolonged erections; and signs of circulation problems in fingers or toes (numbness, pain, color changes).
Very high doses or misuse (for example, taking more than prescribed or using without a prescription) can lead to dangerously high blood pressure, irregular heartbeat, overheating, or sudden death.
Warnings and precautions
Lisdexamfetamine should not be used in people taking or who recently stopped (within 14 days) a monoamine oxidase inhibitor (MAOI), and is generally avoided in patients with serious heart disease, certain structural heart abnormalities, or uncontrolled high blood pressure.
Use requires caution and close monitoring in people with a history of psychosis, bipolar disorder, severe anxiety, tics or Tourette syndrome, or substance use disorders, as it can worsen these conditions or be misused.
In children, long‑term use can slow growth (height and weight), so growth should be tracked regularly and treatment plans periodically re‑evaluated.
During pregnancy and breastfeeding, the decision to use lisdexamfetamine balances potential maternal benefits against possible risks such as low birth weight, preterm delivery, or effects in the nursing infant; individualized medical supervision is important.
It is not approved for ADHD in children under 6 years or for binge eating disorder in anyone under 18 years, and older adults may be more sensitive to cardiovascular and appetite‑suppressing effects.
Relative safety compared with similar drugs
Overall safety risks (cardiovascular, psychiatric, growth, and misuse potential) are comparable to other amphetamine‑type stimulants; its prodrug design may reduce rapid euphoria if tampered with, but it still carries a significant risk of abuse and dependence.
Reporting side effects and finding safety updates
Side effects can be reported to the prescribing clinician and to the FDA MedWatch program by phone or online, and up‑to‑date safety alerts, boxed warnings, and prescribing information are available through the FDA and the manufacturer’s medication guide.
Interactions and Precautions
Major drug interactions
Lisdexamfetamine must not be used with monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping an MAOI due to the risk of dangerously high blood pressure and other serious reactions.
Combining with other stimulant medicines, some decongestants, or weight‑loss products can excessively raise heart rate and blood pressure, while serotonergic drugs (such as many antidepressants, certain pain medicines, and St. John’s wort) can increase the risk of serotonin syndrome.
Certain acidifying or alkalinizing agents (for example, some antacids or urinary pH‑altering drugs) and strong CYP2D6 inhibitors may change amphetamine levels in the body, potentially requiring closer monitoring.
Interactions with OTC drugs, supplements, food, and alcohol
Over‑the‑counter cold and allergy remedies containing pseudoephedrine or phenylephrine, as well as high‑caffeine products, can add to stimulant effects on the heart and blood pressure.
Alcohol can increase cardiovascular and mental health risks and impair judgment, so patients are usually advised to limit or avoid drinking while taking lisdexamfetamine.
Food does not markedly change overall exposure, although a high‑fat meal may slightly delay onset; the medication can be taken with or without food in a consistent way that works for the patient.
Precautions and conditions requiring caution
Careful evaluation is needed in people with structural heart disease, arrhythmias, moderate to severe hypertension, hyperthyroidism, glaucoma, severe anxiety, psychotic disorders, bipolar disorder, tics or Tourette syndrome, and those with a history of substance use disorders.
In kidney disease, dose adjustments and monitoring are important; in significant liver disease, closer observation is recommended even though formal adjustment guidelines are limited.
Monitoring needs
Before and during treatment, clinicians typically monitor blood pressure, heart rate, weight, and, for children, height and growth trajectory.
Patients should also be observed for mood or behavior changes, new or worsening anxiety, psychosis, or manic symptoms, as well as for signs of misuse, abuse, or diversion.
Urine drug screens may detect amphetamines while taking lisdexamfetamine, which should be communicated to testing providers to avoid misinterpretation.
Common Questions and Answers
Q: How long does lisdexamfetamine last during the day?
A: In most people, a single morning dose provides noticeable effects for about 10–14 hours, with some variation depending on dose, individual metabolism, and sensitivity.
Q: Is lisdexamfetamine the same as Adderall?
A: Both are stimulant medications, but lisdexamfetamine is a prodrug that turns into dextroamphetamine only after absorption, while Adderall contains a mixture of amphetamine salts; many people respond similarly, but some find one works better or has fewer side effects than the other.
Q: Will I lose weight on lisdexamfetamine?
A: Decreased appetite and modest weight loss are common, especially when starting or increasing the dose, but the medication is not approved or recommended for weight loss, and significant or unwanted weight changes should be discussed with your clinician.
Q: Can I stop lisdexamfetamine suddenly?
A: Stopping abruptly usually does not cause dangerous withdrawal but can lead to fatigue, low mood, and a return of ADHD or binge‑eating symptoms, so changes are best done in consultation with the prescriber, sometimes with gradual dose adjustments.
Q: Is lisdexamfetamine addictive?
A: Like other amphetamine stimulants, lisdexamfetamine is a Schedule II controlled substance with a risk of misuse and dependence, especially if taken in higher doses, more often than prescribed, or without a prescription, which is why careful monitoring and secure storage are important.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage
Store at room temperature (about 68–77°F / 20–25°C), away from excess heat, moisture, and light, and keep the medication in its original, tightly closed container.
Because lisdexamfetamine is a Schedule II controlled substance, always keep it in a secure place out of sight and reach of children, teenagers, and anyone for whom it is not prescribed, and do not share it with others.
Disposal
Use a community drug take‑back program or authorized drug collection site when possible for unused or expired capsules or chewable tablets.
If no take‑back option is available, mix the medication (do not crush chewables further) with an undesirable substance such as used coffee grounds or cat litter, place it in a sealed bag or container, and throw it in the household trash after scratching out personal information on the prescription label.
Do not flush lisdexamfetamine down the toilet or sink unless the product label or local guidance specifically instructs you to do so.