At a Glance
How It Works
- Methylphenidate is a stimulant that increases levels of the brain chemicals dopamine and norepinephrine, which help improve attention, focus, and impulse control.
- It works mainly by blocking the reuptake (reabsorption) of these chemicals in certain brain areas so they stay active longer.
- In people with ADHD, this often leads to better concentration, less hyperactivity, and improved ability to organize and complete tasks.
Treatment and Efficacy
Approved indications
- Oral methylphenidate hydrochloride is FDA-approved primarily for the treatment of ADHD in children (often starting at age 6), adolescents, and adults, and some formulations are also approved for the treatment of narcolepsy in adults.
- Different brands and release formulations (immediate-release, sustained-release, extended-release) may have specific age ranges and indications on their labels.
Off-label uses and evidence
- Clinicians may sometimes prescribe methylphenidate off-label for conditions such as treatment-resistant depression, certain fatigue syndromes, or cognitive symptoms in neurologic conditions; evidence for these uses ranges from limited to moderate and is not as robust as for ADHD.
- Off-label use is typically reserved for specialized settings when standard treatments have been inadequate and is closely monitored.
Efficacy expectations and onset
- For ADHD, many people notice improvement in attention and hyperactivity/impulsivity within the first day of an effective dose, with full benefit emerging over days to weeks as the dose is adjusted.
- Typical clinical outcomes include improved focus, reduced restlessness, better school or work performance, and improved ability to complete tasks; not everyone responds, and some require alternative stimulants or non-stimulant medications.
- For narcolepsy, methylphenidate can reduce excessive daytime sleepiness, often with noticeable benefit soon after an effective dose is reached.
Comparison to similar drugs
- Efficacy for ADHD is generally comparable to other stimulant medications such as amphetamine formulations, though individual response and side-effect profiles vary.
- Some patients respond better or tolerate methylphenidate more easily than amphetamines, while others have the opposite experience; trials with more than one stimulant class are common in clinical practice.
Dosage and Administration
Typical dosing and how to take
- For ADHD, children often start with low doses of immediate-release methylphenidate (for example, 5 mg once or twice daily) taken before breakfast and lunch, with gradual increases based on response and tolerability; adults may start at slightly higher doses (such as 10 mg daily in divided doses).
- Extended-release formulations are usually taken once each morning and are designed to provide coverage throughout the school or work day; they should generally be swallowed whole and not crushed or chewed unless the product labeling specifically allows opening and sprinkling.
- It may be taken with or without food, but taking it at the same time each day and avoiding doses late in the afternoon or evening can help reduce insomnia.
Special dosing instructions
- Dosing is individualized, with regular follow-up visits to adjust the amount and timing and to assess benefits and side effects.
- Some people may use "drug holidays" (such as weekends or school breaks) under clinician guidance to reassess the need for treatment or to help manage growth and appetite concerns in children.
- Do not change the dose, stop suddenly, or switch between different methylphenidate products without consulting the prescriber, as formulations are not always interchangeable on a milligram-to-milligram basis.
Missed dose guidance
- If a dose is missed, take it when remembered unless it is late in the day; if it is close to the next scheduled dose or near bedtime, skip the missed dose to avoid insomnia and do not double doses.
- Maintain regular dosing times and use reminders if needed.
Overdose guidance
- In case of suspected overdose, seek emergency medical help or contact a poison control center immediately, even if symptoms are mild.
- Symptoms of overdose may include severe restlessness, tremor, confusion, vomiting, sweating, rapid heartbeat, chest pain, very high blood pressure, hallucinations, or seizures and require urgent evaluation.
Safety and Side Effects
Common side effects
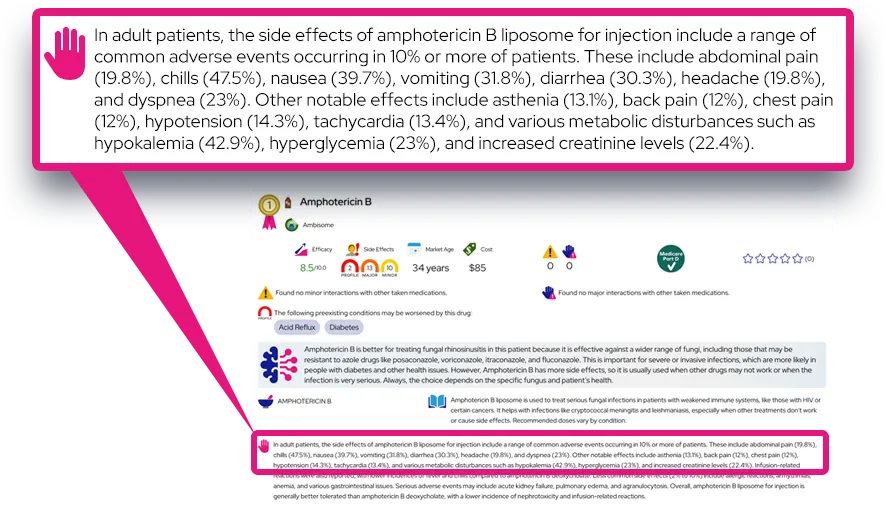
- Common side effects include decreased appetite, weight loss, trouble sleeping, dry mouth, headache, stomachache, nausea, and increased heart rate or blood pressure.
- These effects often begin soon after starting the medicine or after a dose increase and may lessen as the body adjusts or with dose or schedule changes.
- Many people tolerate the medication well at the lowest effective dose, but ongoing monitoring of appetite, weight, sleep, and mood is recommended.
Serious or rare adverse effects
- Serious reactions that require immediate medical attention include chest pain, shortness of breath, fainting, irregular heartbeat, signs of stroke, sudden vision changes, severe or new psychiatric symptoms (such as hallucinations, mania, or aggressive behavior), and signs of allergic reaction (rash, swelling, trouble breathing).
- Rare but serious problems include sudden cardiac events in people with underlying heart disease, severe high blood pressure, prolonged painful erections (priapism), and circulation problems in fingers or toes (changes in color, numbness, pain).
Warnings and precautions
- Methylphenidate is generally not recommended for people with known serious structural heart disease, certain heart rhythm problems, or uncontrolled high blood pressure, unless carefully evaluated by a specialist.
- Use with caution in people with a history of psychiatric conditions such as bipolar disorder, psychosis, severe anxiety, or substance use disorders, as symptoms can worsen or misuse may occur.
- During pregnancy, use is considered only if the potential benefits justify potential risks; breastfeeding decisions should be individualized with a clinician because small amounts can pass into breast milk.
- Dose adjustments and close monitoring may be needed in people with significant liver or kidney disease.
Comparative safety
- Overall safety is similar to other stimulant medications: effective but with known risks for appetite suppression, cardiovascular effects, and potential for misuse or dependence.
- Non-stimulant ADHD medications may be preferred for some people with certain heart, psychiatric, or substance use conditions, or when stimulants are not tolerated.
Reporting side effects and safety updates
- Patients and caregivers can report suspected side effects to their prescriber and to the FDA’s MedWatch program or equivalent national reporting systems.
- Updated safety communications, boxed warnings, and prescribing information are available through official regulatory websites and medication guides for each specific product.
Interactions and Precautions
Drug and substance interactions
- Methylphenidate can interact with monoamine oxidase inhibitors (MAOIs) and should not be used with them or within a recommended washout period because of the risk of dangerously high blood pressure and other serious reactions.
- It may also interact with certain blood pressure medications, other stimulants (including some decongestants and weight-loss products), some antidepressants, and drugs that affect heart rhythm, which can alter blood pressure, heart rate, or arrhythmia risk.
- Alcohol can increase side effects and, with some extended-release formulations, may affect how the drug is released; alcohol use should generally be limited or avoided.
- Patients should tell their clinician about all prescription medicines, over-the-counter products (such as cold medicines), and supplements (including herbal stimulants like caffeine-containing products or ginseng).
Precautions and conditions requiring care
- Use requires particular caution in people with a history of cardiovascular disease, high blood pressure, hyperthyroidism, glaucoma, severe anxiety, tics or Tourette syndrome, bipolar disorder, psychotic disorders, or substance use disorders.
- A careful medical history, including family history of sudden cardiac death or arrhythmias, is recommended before starting, and many clinicians check baseline blood pressure, heart rate, and sometimes an ECG in at-risk patients.
Monitoring needs
- Regular monitoring typically includes checks of blood pressure, heart rate, weight, height (in children), appetite, sleep, and mood or behavior changes.
- Additional tests, such as ECGs or lab tests, may be ordered for individuals with specific medical conditions or risk factors, or if concerning symptoms develop.
Common Questions and Answers
Q: How long does it take for oral methylphenidate to start working for ADHD?
A: Immediate-release forms usually begin working within 30–60 minutes, and extended-release forms within a couple of hours, with noticeable improvements often seen the same day once the right dose is reached.
Q: Will methylphenidate change my personality?
A: When properly dosed, it should improve focus and self-control without changing who you are; if you feel "flat," overly quiet, or unlike yourself, the dose or formulation may need adjustment.
Q: Do I have to take methylphenidate every day?
A: Many people take it daily for consistent symptom control, but some use it mainly on school or work days; any changes to the schedule, including "drug holidays," should be planned with your clinician.
Q: Is methylphenidate addictive?
A: Methylphenidate has a potential for misuse and dependence, especially when taken at higher-than-prescribed doses or without a prescription, but when used as directed and carefully monitored, the risk of addiction in people with ADHD is lower.
Q: Can children outgrow the need for methylphenidate?
A: Some children’s ADHD symptoms lessen with age and they may no longer need medication, while others continue to benefit into adulthood; periodic reevaluation with the clinician helps determine whether treatment should be continued, changed, or stopped.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage
- Store methylphenidate at room temperature, away from excess heat, moisture, and direct light, in its original, tightly closed container and out of reach of children and pets.
- Keep track of the number of tablets or capsules, as it is a controlled substance with potential for misuse.
Disposal
- Do not save leftover methylphenidate for future use or share it with others; this is unsafe and illegal.
- Use a drug take-back program or authorized collection site when possible; if unavailable, follow local guidance, which often includes mixing unused medicine with an undesirable substance (like used coffee grounds), sealing in a container, and placing it in household trash, unless your pharmacist or medication guide instructs otherwise.