Peramivir
At a Glance

How It Works
- Rapivab is a neuraminidase inhibitor, which means it blocks an enzyme on the flu virus that it needs to spread from cell to cell.
- By stopping new virus particles from being released, it limits how quickly the virus multiplies in your body.
- This helps your immune system clear the infection faster and can shorten the length and severity of flu symptoms when given early.
Treatment and Efficacy
Approved indications: Rapivab is approved as a single-dose intravenous treatment for acute uncomplicated influenza A or B in patients 6 months of age and older whose symptoms have been present for no more than 2 days; it is not approved for flu prevention or for treating severe influenza in patients who require hospitalization.
Off-label uses: Clinicians may use peramivir off-label in hospitalized or critically ill patients with influenza, or when oral or inhaled antivirals (such as oseltamivir or zanamivir) cannot be used, but most data in these settings come from small studies, case series, and outbreak experience rather than large randomized trials, so the evidence is considered limited to moderate.
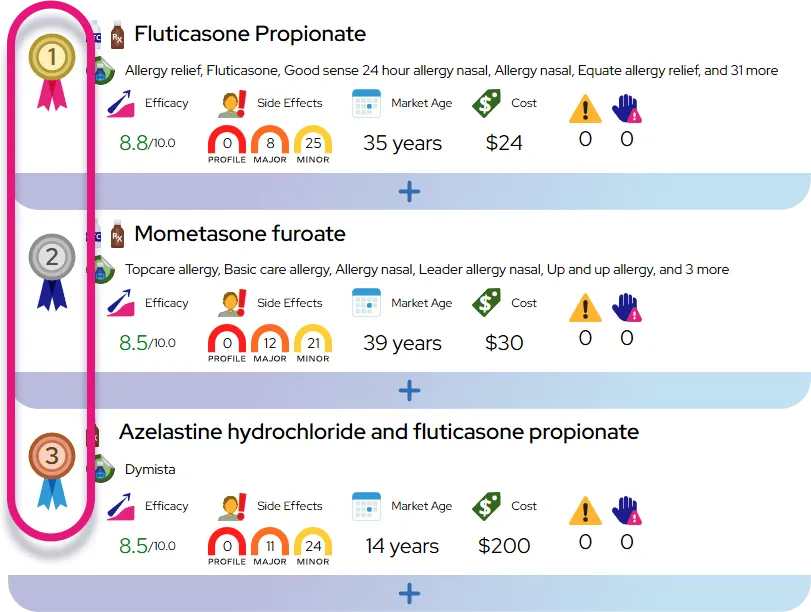
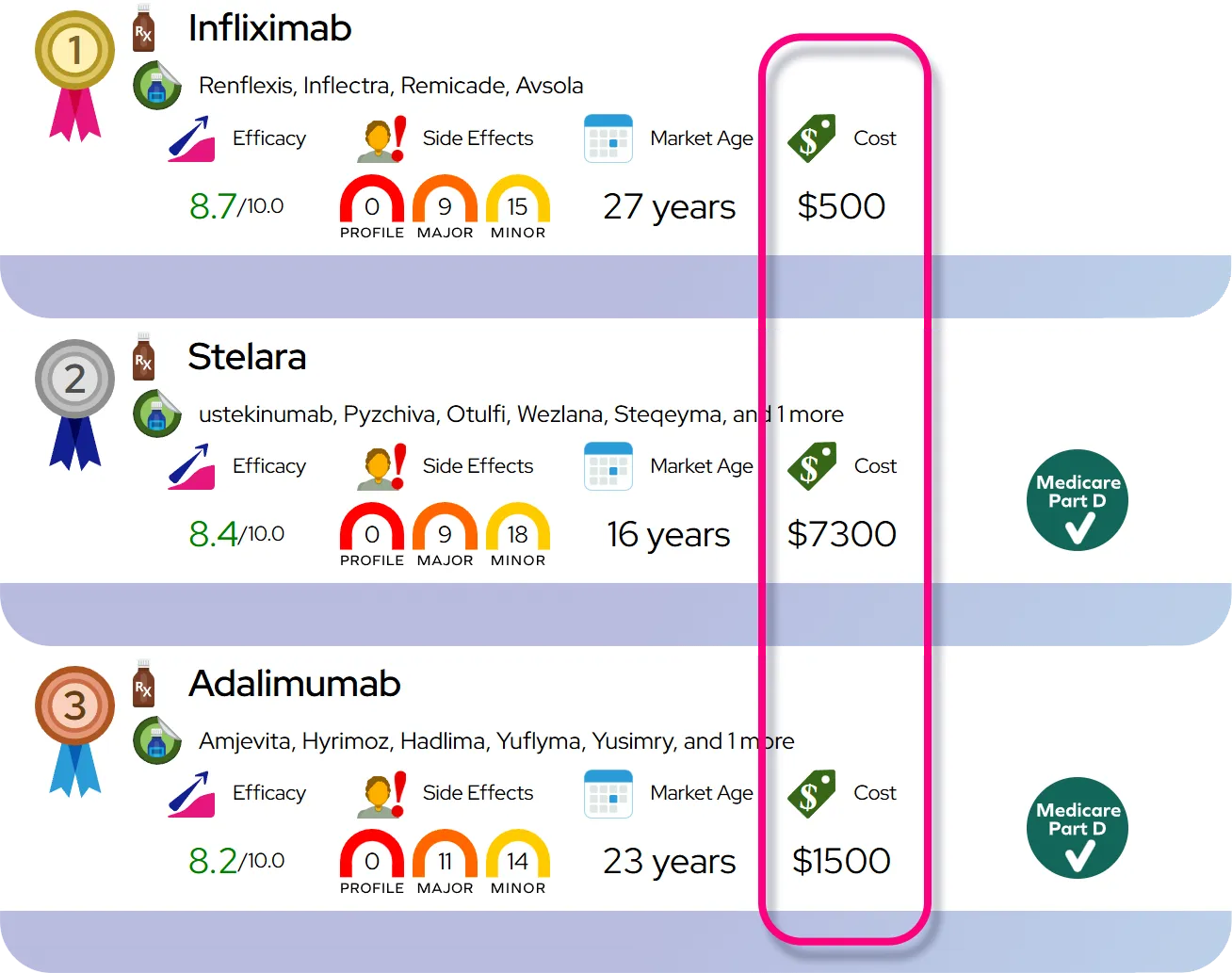
Efficacy expectations: When started within 48 hours of symptom onset, Rapivab typically shortens time to improvement of flu symptoms by roughly a day compared with no antiviral, helps fever and viral shedding resolve sooner, and produces clinical outcomes that are generally similar to those seen with other neuraminidase inhibitors, with its main advantage being a convenient single IV dose rather than clearly superior effectiveness.
Dosage and Administration
Typical dosing by age: For acute uncomplicated influenza, adults and adolescents 13 years and older receive a single 600 mg dose of Rapivab by intravenous infusion over 15–30 minutes, and children 6 months to 12 years receive a single 12 mg/kg dose (up to a maximum of 600 mg) infused over 15–30 minutes; treatment should begin as soon as possible and no later than 2 days after symptoms start.
Dose adjustments and special instructions: In adults and adolescents with creatinine clearance 30–49 mL/min, the dose is reduced to 200 mg, and with creatinine clearance 10–29 mL/min to 100 mg; in children 2–12 years with creatinine clearance 30–49 mL/min the dose is 4 mg/kg and with 10–29 mL/min it is 2 mg/kg (up to 600 mg), and in patients on chronic hemodialysis an appropriately reduced single dose is given after dialysis, while dosing in infants 6 months to less than 2 years with significant renal impairment is not well defined and requires specialist judgment.
How the medicine is given: Rapivab comes as a solution that is diluted into a compatible IV fluid and infused through a vein in a clinic or hospital; it is not taken by mouth, does not depend on meals, and the entire course of treatment is completed with this one infusion.
Missed dose and overdose: Because Rapivab is generally given as a one-time infusion under medical supervision, missing a dose usually means a missed appointment and should be addressed by contacting the treating healthcare team promptly, and any suspected overdose is managed in a medical setting with monitoring and supportive care, with the possibility of hemodialysis to help remove the drug because it is cleared mainly by the kidneys.
Safety and Side Effects
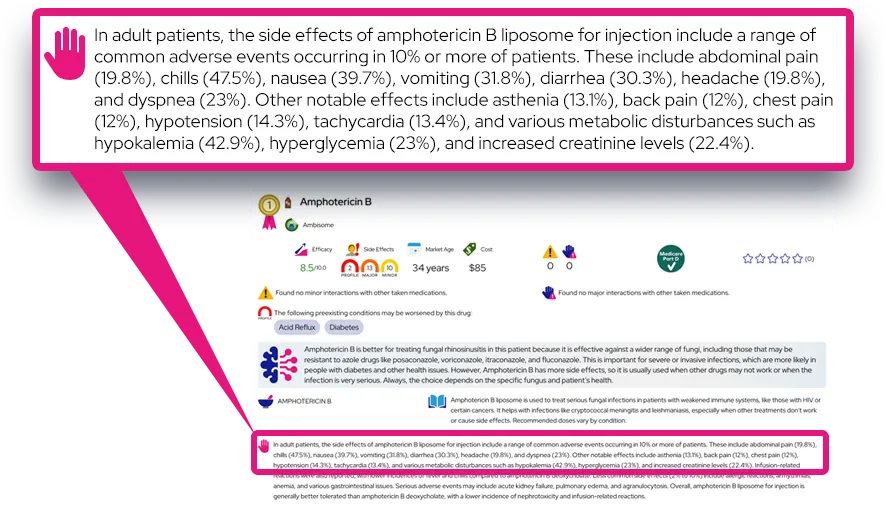
Common side effects: The most common side effect is diarrhea, and others such as nausea, vomiting (more often in children), constipation, or trouble sleeping can occur; these usually start soon after the infusion, are mild to moderate, and resolve on their own.
Serious or rare adverse effects: Rare but important reactions include severe allergic reactions (including anaphylaxis), serious skin reactions such as Stevens–Johnson syndrome or erythema multiforme, and neuropsychiatric events like confusion, hallucinations, or abnormal behavior, which require immediate medical attention.
Warnings and precautions: Rapivab should not be used in anyone with a known serious allergy to peramivir or any component of the product; dose reduction is needed in patients with significant kidney impairment, while liver disease usually does not require adjustment, and there are limited data in pregnancy and breastfeeding so use is generally reserved for situations where the expected benefit outweighs potential risks.
Safety compared with similar drugs: Overall, Rapivab’s safety profile is similar to other neuraminidase inhibitors, with gastrointestinal symptoms being the most common effects and serious skin or neuropsychiatric reactions reported rarely; the IV route means it is given under direct healthcare supervision, which allows rapid management of any infusion-related reactions.
Side-effect reporting and safety updates: Side effects can be reported to the U.S. Food and Drug Administration (FDA) through the MedWatch program (by phone at 1-800-FDA-1088 or online) or to the manufacturer using the phone number on the medication information, where the latest safety communications are also reflected in updated prescribing information.
Interactions and Precautions
Drug and vaccine interactions: The main clinically important interaction is with live attenuated intranasal influenza vaccines (such as the nasal spray flu vaccine), whose effectiveness can be reduced if given within 2 weeks before or 48 hours after Rapivab; inactivated (injected) flu vaccines and most other prescription or over-the-counter medicines have no known significant interactions with Rapivab, though a full medication review is still recommended.
Other products, food, and alcohol: No specific interactions are known with foods, alcohol, vitamins, or herbal supplements, but heavy alcohol use or unregulated supplements may complicate recovery from flu or interact with other medicines being used at the same time.
Precautions and monitoring: Use Rapivab cautiously in patients with kidney disease, adjusting the dose according to renal function and monitoring creatinine clearance as needed; during and after the IV infusion, patients are typically observed for changes in blood pressure, breathing, skin reactions, or new confusion or abnormal behavior, while routine laboratory or ECG monitoring is generally limited to assessing kidney function and the overall clinical status of sicker patients.
Common Questions and Answers
Q: What is Rapivab and how is it given?
A: Rapivab is an antiviral medicine for treating the flu that is given as a single intravenous infusion in a clinic or hospital rather than as pills or an inhaled medicine.
Q: How soon will I feel better after receiving Rapivab?
A: Many people start to notice improvement in fever and other flu symptoms within about a day after the infusion, especially when treatment is started within 48 hours of symptoms beginning, though the exact response varies from person to person.
Q: Does Rapivab replace the flu shot?
A: No, Rapivab is used to treat flu after you are already sick and does not provide long-term protection, so annual influenza vaccination is still recommended as the main way to prevent flu.
Q: Can children and older adults receive Rapivab?
A: Yes, Rapivab is approved for children 6 months and older and can be used in older adults as well, with dosing adjusted by age and kidney function.
Q: Is Rapivab safe during pregnancy or while breastfeeding?
A: Human data are limited, so Rapivab is generally used in pregnancy or breastfeeding only when the potential benefits of treating flu are judged to outweigh possible risks, and other antivirals with more experience may be preferred when appropriate.
Q: Will I still be contagious after getting Rapivab?
A: Rapivab can help reduce the amount of virus in your body and may shorten the time you are contagious, but you can still spread flu for a period of time, so standard precautions like staying home while sick, covering coughs, and good hand hygiene remain important.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →