At a Glance
How It Works
- Dexmethylphenidate is a stimulant that increases levels of natural brain chemicals (dopamine and norepinephrine) involved in attention and impulse control.
- It mainly works by blocking the reuptake (reabsorption) of these chemicals so more stays available between nerve cells.
- This helps people with ADHD focus better, stay on task longer, and reduce hyperactive or impulsive behavior while the medicine is active.
Treatment and Efficacy
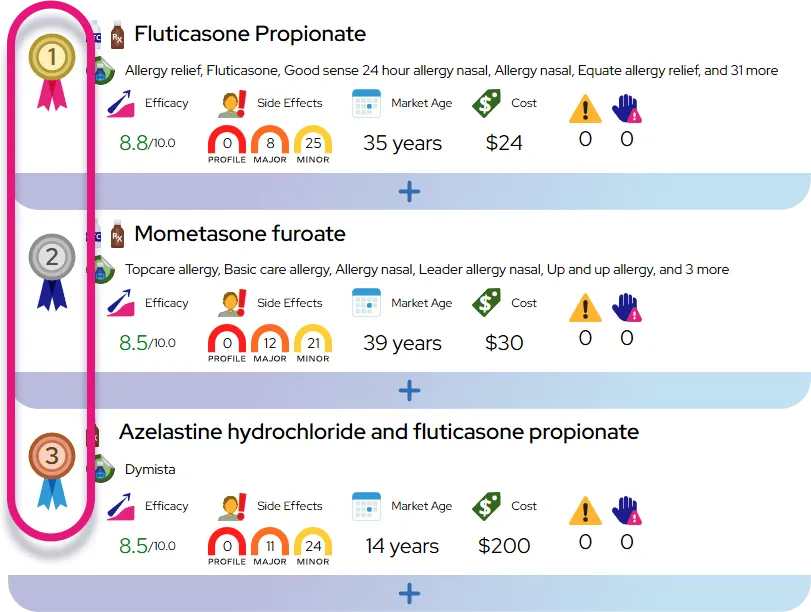
Approved indications: Dexmethylphenidate hydrochloride (immediate-release and extended-release) is FDA-approved for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children, adolescents, and adults 6 years and older.
Off-label uses: Clinicians may occasionally prescribe dexmethylphenidate off-label for conditions such as narcolepsy or fatigue related to certain medical conditions, usually based on experience with methylphenidate-class stimulants; evidence for these uses is more limited and typically consists of smaller studies or extrapolation rather than large, dedicated trials.
Onset and time course: Immediate-release tablets usually begin working within about 30 to 60 minutes and last around 4 to 6 hours; extended-release capsules typically start to work within 1 to 2 hours and may control symptoms for about 8 to 12 hours, depending on the individual.
Expected benefits: Many patients experience improved attention, decreased distractibility, better task completion, and reduced hyperactivity/impulsivity on school, work, or home activities; improvements often appear on the first treatment day but may require several weeks of dose adjustments to optimize effect.
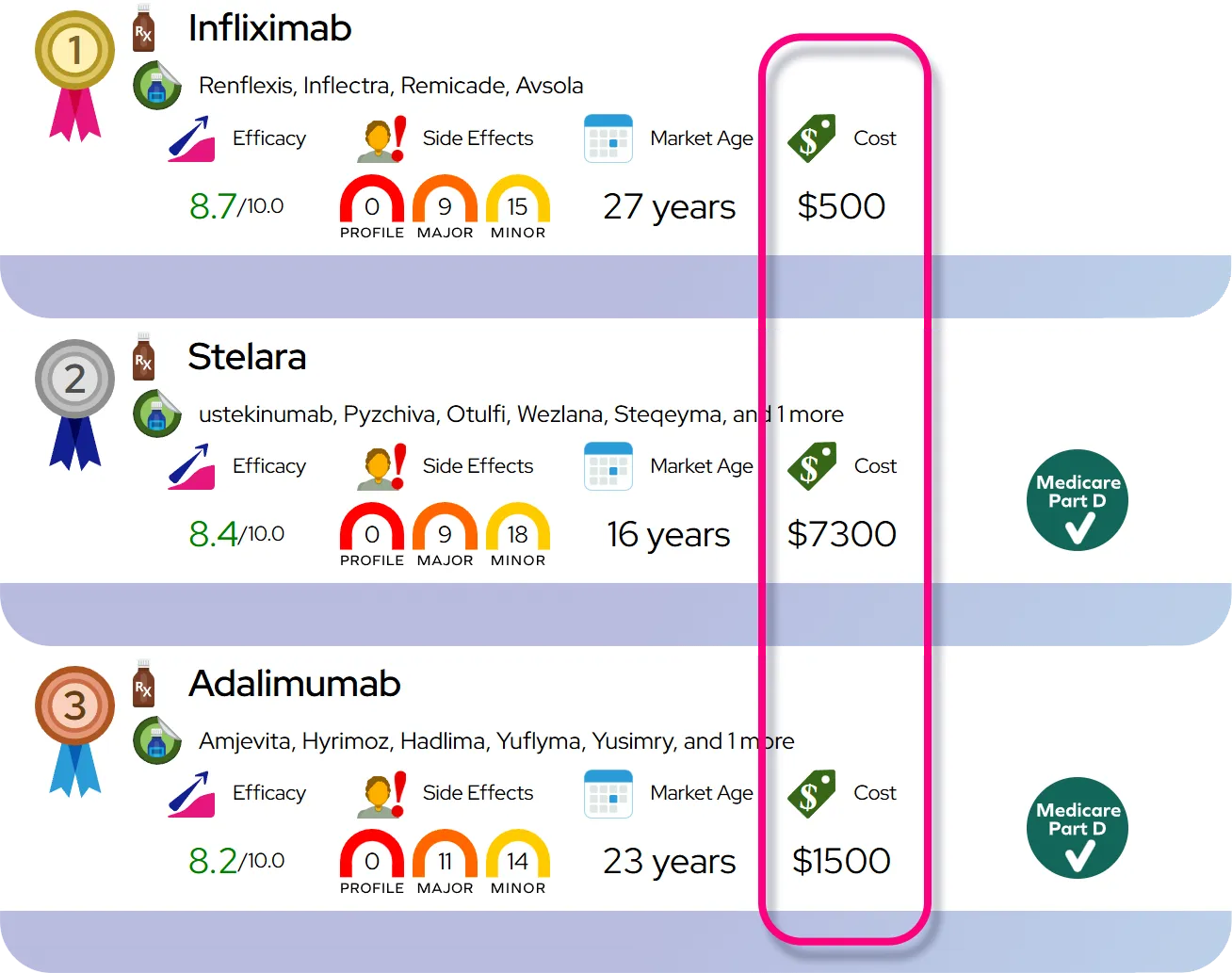
Response rates and comparison: As with other stimulants, a substantial proportion of people with ADHD (often cited around two-thirds) obtain meaningful benefit, while some have partial or minimal response; overall efficacy is comparable to racemic methylphenidate and other stimulant options, so choice among them usually depends on side effects, duration of action, prior response, and patient preference.
Dosage and Administration
Typical dosing (immediate-release tablets): For children, adolescents, and adults 6 years and older who are new to methylphenidate, a common starting dose is 2.5 mg by mouth twice daily at least 4 hours apart (for example, morning and around lunchtime), with weekly increases of 2.5 to 5 mg per day as needed; the usual maximum is 10 mg twice daily (20 mg per day).
Typical dosing (extended-release capsules): For children and adolescents 6 years and older not already taking stimulants, a usual starting dose is 5 mg by mouth once each morning; for adults, 10 mg by mouth once each morning; doses can be increased in 5 mg (children) or 10 mg (adults) steps about weekly based on response and tolerability, generally up to 30 mg per day in pediatric patients and 40 mg per day in adults.
Switching from other stimulants: When changing from racemic methylphenidate, the total daily dose of dexmethylphenidate is typically about half the total daily methylphenidate dose; when switching from immediate-release dexmethylphenidate to extended-release, the same total daily milligram dose is usually given once each morning as the extended-release form.
How to take it: Tablets and capsules are taken by mouth and may be taken with or without food; extended-release capsules should be swallowed whole or opened and the contents sprinkled on a small amount of applesauce and swallowed without chewing, and the beads should not be crushed, chewed, or divided; to reduce insomnia, doses are usually given in the morning (and midday for twice-daily tablets) and avoided in the late afternoon or evening.
Special dosing instructions: Before starting, clinicians often check heart history, blood pressure, heart rate, and psychiatric history, and during treatment they typically monitor blood pressure, pulse, appetite, weight (especially in children), and ADHD symptoms; the dose should be the lowest that provides effective symptom control, and periodic breaks or reassessments may be used to confirm continued need.
Missed dose guidance: If a once-daily morning dose is missed and it is still relatively early in the day, it may be taken when remembered; if it is late in the day, the dose is usually skipped to avoid insomnia, and the regular schedule is resumed the next day; for immediate-release tablets, a missed dose near the usual second dose time is sometimes taken if not too late in the afternoon, but doses should not be doubled to “make up” for a missed dose.
Overdose: Taking too much can cause vomiting, agitation, tremor, confusion, sweating, very fast heartbeat, high blood pressure, hallucinations, or seizures; in any suspected overdose, emergency medical help or a poison control center should be contacted immediately, and no additional doses should be taken.
Safety and Side Effects
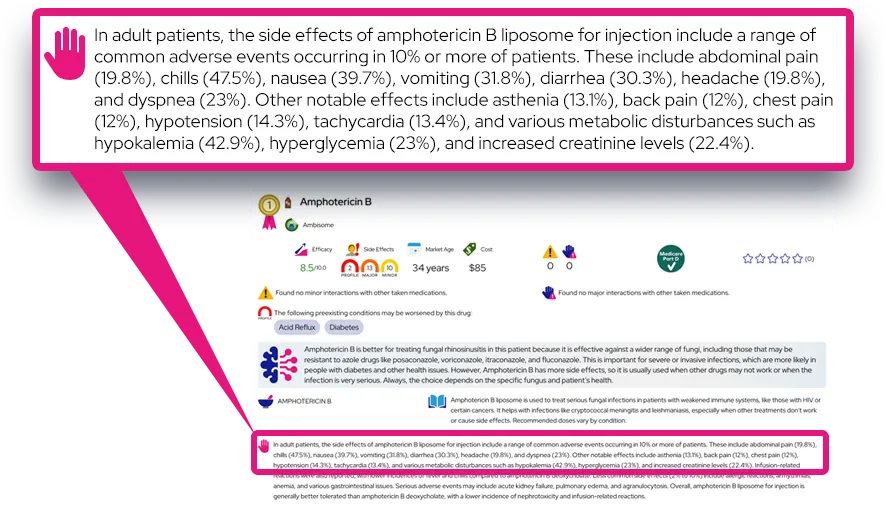
Common side effects: Frequently reported effects include decreased appetite, weight loss over time, stomach pain, nausea, dry mouth, headache, trouble sleeping, nervousness or anxiety, and increased heart rate or blood pressure; these often begin soon after starting or increasing the dose and may lessen as the body adjusts or with dose or schedule changes.
Serious or rare adverse effects: Rare but important risks include chest pain, shortness of breath, fainting, or signs of heart problems; new or worsening aggression, irritability, psychosis (such as hallucinations), or mania; seizures in susceptible patients; new or worsening tics or Tourette’s syndrome; prolonged or painful erections (priapism); and circulation problems in fingers or toes (color changes, pain, or numbness) that may indicate peripheral vasculopathy or Raynaud’s phenomenon; any of these require prompt medical attention.
Warnings and precautions: Dexmethylphenidate is not recommended in people with known serious structural heart disease, serious arrhythmias, or moderate-to-severe high blood pressure, and should be used cautiously in those with cardiovascular risk factors; it can worsen anxiety, agitation, bipolar disorder, psychosis, or tics, so careful psychiatric assessment and monitoring is advised; in children, long-term use may be associated with slowed growth, so height and weight should be tracked regularly.
Pregnancy and breastfeeding: Data in pregnancy are limited; the medicine is generally used only when potential benefits justify possible risks, and alternative approaches may be considered; small amounts can appear in breast milk, so the decision to use while breastfeeding is individualized, balancing maternal benefit with potential infant exposure and monitoring for irritability, poor feeding, or sleep changes.
Age considerations and organ function: Safety and effectiveness are not established in children under 6 years, and use in this age group is not recommended; in older adults, cardiovascular screening and cautious dosing are important; no specific kidney or liver dose adjustments are defined, but caution is reasonable in significant hepatic or renal impairment because formal studies are limited.
Relative safety compared with similar drugs: Overall safety profile is similar to other methylphenidate-containing stimulants, with comparable risks of appetite loss, sleep disturbance, cardiovascular effects, and abuse or dependence; as the d-enantiomer of methylphenidate, dexmethylphenidate is generally used at about half the total milligram dose of racemic methylphenidate for a similar clinical effect.
Reporting side effects and staying updated: Suspected side effects can be reported to a health professional and to the FDA MedWatch program (by phone or online), and patients should review the current Medication Guide and any new safety communications that accompany their prescription at the pharmacy.
Interactions and Precautions
Major drug interactions: Dexmethylphenidate must not be used with monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping them due to risk of dangerous blood pressure elevation; it can reduce the effect of blood pressure medicines, and combining it with other stimulants or decongestants (such as pseudoephedrine or phenylephrine) can further increase heart rate and blood pressure; it may interact with certain seizure medicines, tricyclic antidepressants, and warfarin, sometimes requiring dose adjustment and monitoring.
Other medicines, supplements, and substances: Combining with other medicines that affect the brain (such as some antidepressants, antipsychotics, or mood stabilizers) requires careful supervision to watch for mood or blood pressure changes; alcohol can interfere with the release profile of some extended-release capsules and may worsen side effects, so it is generally advised to avoid alcohol while taking this medication; patients should also inform their clinician about herbal products and supplements, including those with stimulant or caffeine-like effects.
Food and diagnostic procedures: Dexmethylphenidate can be taken with or without food, though taking with food may lessen stomach upset for some people; on days when halogenated inhalation anesthetics are planned for surgery, stimulants are often withheld due to possible heart rhythm risks, according to anesthesiology and labeling precautions.
Conditions requiring caution or avoidance: Use is typically avoided in people with serious structural heart disease, cardiomyopathy, serious arrhythmias, or significant uncontrolled hypertension; it is generally contraindicated in individuals with glaucoma, severe anxiety or agitation, or a history of certain motor or vocal tics or Tourette’s syndrome without careful evaluation; people with a history of substance use disorder require close monitoring because of the potential for misuse, abuse, and dependence.
Monitoring needs: Before and during treatment, clinicians usually monitor blood pressure and heart rate, appetite, weight, and in children, growth (height and weight) over time; psychiatric status is monitored for mood changes, irritability, tics, or psychotic symptoms; some prescribers may periodically check blood counts or obtain an ECG in patients with cardiac risk factors based on individual circumstances.
Common Questions and Answers
Q: How long does dexmethylphenidate work each day?
A: Immediate-release tablets usually last about 4 to 6 hours per dose, while extended-release capsules are designed to control symptoms for most of the school or work day, often around 8 to 12 hours depending on the person and the dose.
Q: When will I notice improvement in my ADHD symptoms?
A: Many people notice some benefit the first day they take an effective dose, but it can take several days to weeks of careful dose adjustments to find the dose that gives the best balance of symptom control and side effects.
Q: Can I drink coffee or energy drinks while taking this medication?
A: Caffeine and other stimulants can add to side effects such as jitteriness, rapid heartbeat, or trouble sleeping, so moderate or lower intake is usually recommended, and any concerns should be discussed with the prescribing clinician.
Q: Is dexmethylphenidate addictive?
A: Dexmethylphenidate is a controlled substance and can be misused, but when taken exactly as prescribed and monitored by a clinician, it is used safely by many people; those with a history of substance use problems require especially careful supervision.
Q: Will this medicine stunt my child’s growth?
A: Some children on long-term stimulant treatment may grow more slowly in height or weight, especially early in treatment, so health care providers usually track growth regularly and may adjust dosing, timing, or treatment plans if meaningful growth effects are seen.
Q: Can I stop taking dexmethylphenidate suddenly?
A: Dexmethylphenidate does not typically cause dangerous withdrawal when stopped, but symptoms of ADHD may return or feel more noticeable, so changes in dose or discontinuation should be planned with the prescriber rather than done abruptly on your own.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Keep dexmethylphenidate at room temperature (about 68°F to 77°F / 20°C to 25°C), in a dry place away from heat, moisture, and direct light, and in the original child-resistant container.
Safety and security: Because this is a controlled substance with abuse potential, store it in a locked or otherwise secure location, out of sight and reach of children, teenagers, and visitors.
Disposal: When no longer needed, use a medicine take-back program through a pharmacy, clinic, or community collection site when available; if none is available, mix the capsules or tablets (do not crush extended-release beads) with an unwanted substance such as used coffee grounds or cat litter, seal in a bag or container, and place in household trash according to local guidance, and remove or scratch out personal information on prescription labels before throwing away the bottle.