At a Glance
How It Works
Dextroamphetamine sulfate is a central nervous system stimulant that increases certain natural chemicals in the brain to improve focus and wakefulness.- It mainly increases dopamine and norepinephrine, which help with attention, impulse control, and alertness.
- By stimulating specific brain areas that regulate activity and behavior, it can reduce hyperactivity and improve concentration.
- The effects begin within about an hour of a dose and last several hours, depending on whether an immediate- or extended-release form is used.
Treatment and Efficacy
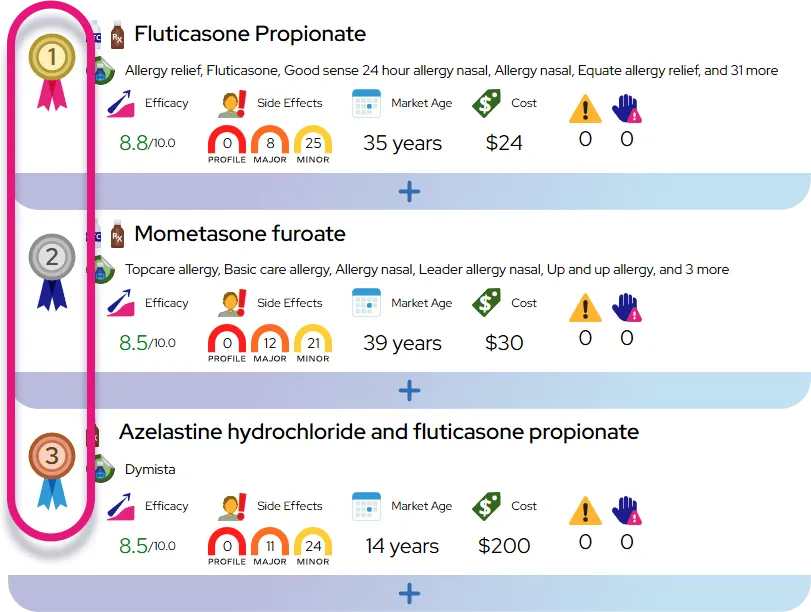
Approved indications: Oral dextroamphetamine sulfate is approved as a central nervous system stimulant for treatment of narcolepsy in adults and pediatric patients and for attention-deficit/hyperactivity disorder (ADHD, historically termed attention deficit disorder with hyperactivity) in children and adolescents; exact age ranges and formulations (immediate- vs extended-release) depend on the specific brand or generic product.
Common off-label uses (evidence varies):
- Adult ADHD, where dextroamphetamine is widely used with evidence showing symptom improvement similar to other stimulant medications.
- Severe fatigue syndromes (for example, related to medical or neurologic illness) and treatment-resistant depression as an augmenting agent, generally supported by small or moderate studies and used when standard options are insufficient.
- Short-term weight loss in obesity has historical use with amphetamines, but this is now uncommon because of safety concerns and availability of safer weight-loss options.
Efficacy expectations:
- Onset: Immediate-release forms usually begin working within about 30–60 minutes and last around 4–6 hours; extended-release forms provide a longer, smoother effect (often much of the waking day).
- ADHD outcomes: Many patients have noticeable improvements in core symptoms (attention, hyperactivity, impulsivity) during the first week at an effective dose, with overall response rates similar to other stimulants (a substantial majority of appropriately selected patients improve).
- Narcolepsy outcomes: Daytime wakefulness and ability to stay alert often improve the same day a therapeutic dose is taken, with ongoing benefit as long as the medication is continued.
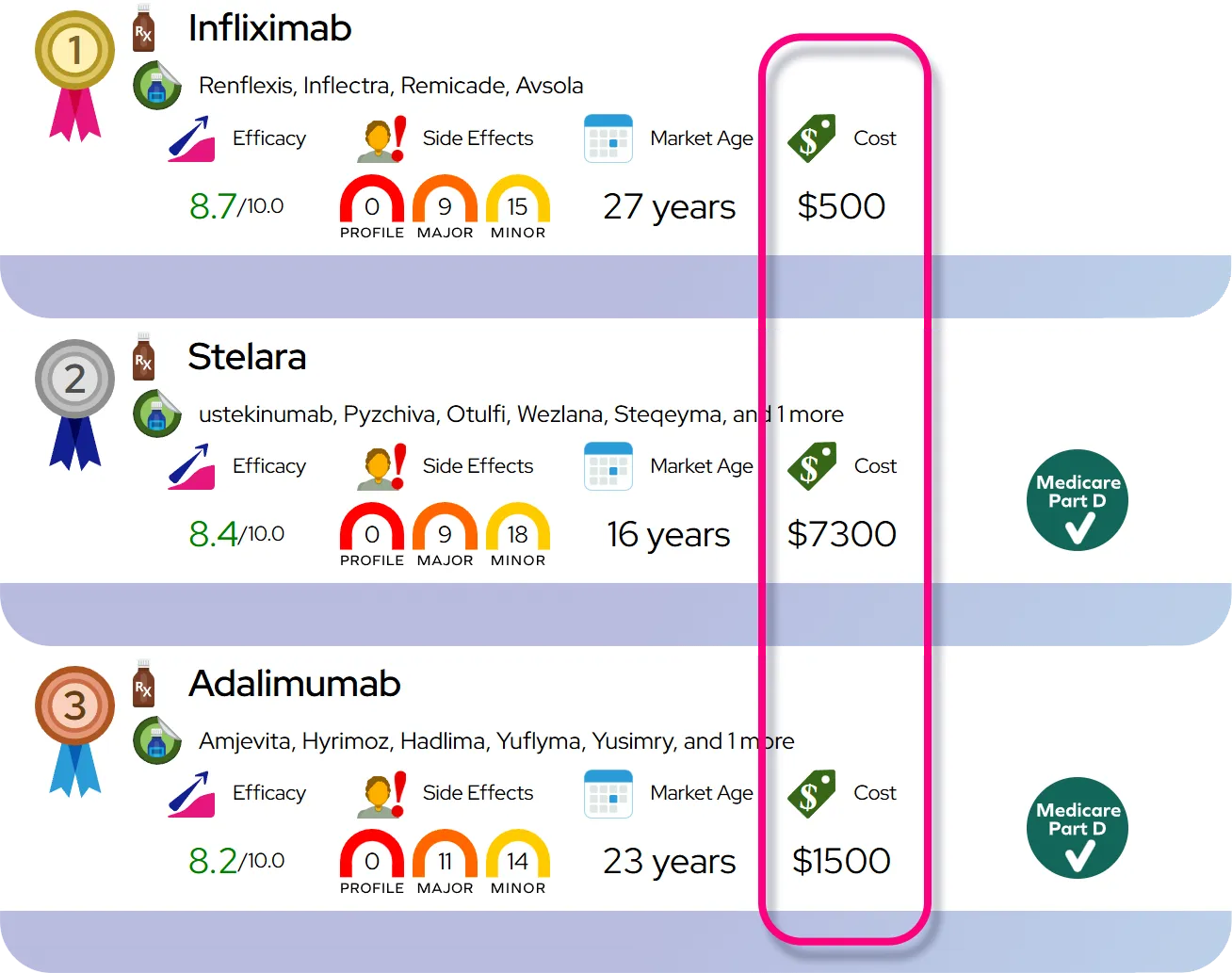
- Comparison to similar drugs: Its effectiveness for ADHD and narcolepsy is broadly comparable to other amphetamine-based stimulants and methylphenidate products; some patients respond better or tolerate one stimulant over another, so choice is often individualized based on effectiveness, side effects, dosing schedule, and personal or family history.
Dosage and Administration
General dosing principles: Doses are individualized and started low, then increased gradually (usually no more often than once weekly) to the lowest amount that controls symptoms; late-day doses are avoided to reduce insomnia.
Typical oral dosing ranges:
- ADHD in children and adolescents: Often begins with 2.5–5 mg once or twice daily of an immediate-release product, with gradual increases; most patients are maintained on a total of about 10–40 mg/day, given in 1–3 doses.
- ADHD in adults (off-label for some products): Similar titration is used, with many adults stabilized between about 10–40 mg/day; some may require higher doses, especially for narcolepsy, under specialist supervision.
- Narcolepsy (adolescents and adults): Typical total daily doses range from 10–60 mg/day, taken in divided doses starting in the morning, adjusted based on wakefulness and tolerability.
How to take it: Take by mouth with a full glass of water, with or without food, at the same times each day; morning dosing (and midday if needed) is preferred to limit sleep problems.
Formulation considerations: Immediate-release tablets or solution are usually taken 1–3 times per day about 4–6 hours apart, while extended-release capsules are taken once daily in the morning; capsules are typically swallowed whole, and any instructions about opening and sprinkling contents should be followed only if explicitly allowed in that product’s directions.
Special dosing instructions: Do not change the dose, stop, or restart the medication without discussing it with a prescriber, as sudden discontinuation from high doses may cause fatigue, low mood, and sleep changes; in some cases, clinicians recommend occasional breaks ("drug holidays") to reassess the need or reduce growth impact in children.
Missed dose guidance: If a dose is missed and it is still early in the day, take it when remembered; if it is near the time for the next dose or late in the afternoon or evening, skip the missed dose and resume at the next scheduled time—do not double up doses.
Overdose: Signs of overdose can include extreme restlessness or agitation, rapid breathing, tremor, confusion, hallucinations, very fast or irregular heartbeat, high fever, or loss of consciousness; in suspected overdose, seek emergency medical care immediately and, in the United States, contact poison control at 1-800-222-1222.
Safety and Side Effects
Common side effects (often dose-related):
- Decreased appetite, weight loss, stomach upset, dry mouth, and nausea.
- Difficulty falling or staying asleep (insomnia), nervousness, restlessness, or feeling "jittery."
- Headache, dizziness, and increased heart rate or blood pressure; these are usually mild but should be monitored.
- In children, slowed weight gain and, less commonly, slowed height growth over time, which is why growth is checked regularly.
Serious or rare adverse effects (seek care immediately):
- Chest pain, shortness of breath, fainting, irregular heartbeat, or signs of stroke (sudden weakness, trouble speaking, vision changes), which may signal heart or blood vessel problems.
- Mood or behavior changes such as new or worsening aggression, severe anxiety, hallucinations, paranoia, or manic symptoms (racing thoughts, decreased need for sleep, extreme mood elevation).
- Signs of poor blood flow to fingers or toes (numbness, pain, color changes such as pale or blue skin), or unexplained wounds on fingers or toes.
- Seizures, very high fever, severe muscle stiffness or twitching, confusion, or agitation, which may indicate serious reactions such as serotonin syndrome when taken with certain other medicines.
- Allergic reactions, including rash, swelling, or trouble breathing.
Warnings and precautions:
- Not appropriate for people with certain heart conditions, significant high blood pressure, advanced hardening of the arteries, severe anxiety or agitation, hyperthyroidism, glaucoma, or those taking monoamine oxidase inhibitors (MAOIs) or who have taken them in the last 14 days.
- Use caution and close supervision in patients with a history of substance use disorder, psychiatric illness (depression, bipolar disorder, psychosis), tics, or Tourette syndrome.
- Pregnancy: Stimulants may affect fetal growth and can cause withdrawal symptoms in the newborn; they are generally used in pregnancy only when benefits clearly outweigh risks.
- Breastfeeding: Dextroamphetamine passes into breast milk and may affect the baby’s sleep, appetite, or growth; many clinicians either avoid use or use the lowest effective dose with monitoring and shared decision-making.
- Age limits: Not recommended in very young children (typically under 3–6 years, depending on product), and older adults may be more sensitive to cardiovascular effects.
- Kidney or liver disease: Severe kidney impairment can increase drug levels and may require dose adjustments and closer monitoring; caution is also advised in significant liver disease.
Relative safety compared with other stimulants: Dextroamphetamine shares class risks with other stimulant medications (potential for abuse and dependence, cardiovascular and psychiatric side effects, growth effects in children); with appropriate screening, dosing, and monitoring, most patients tolerate it well, but it is not inherently safer or riskier than other commonly used stimulants.
Reporting side effects and staying updated: Side effects can be reported to the FDA MedWatch program (online or by phone) or to a healthcare professional, and safety alerts or updated warnings are published on the FDA’s drug safety communication pages and in the prescribing information for each product.
Interactions and Precautions
Major drug and supplement interactions:
- Monoamine oxidase inhibitors (MAOIs): Combining dextroamphetamine with MAOIs (including certain antidepressants and the antibiotics linezolid or IV methylene blue) or using it within 14 days of MAOI therapy can cause dangerous increases in blood pressure and other serious reactions and is contraindicated.
- Other stimulants and sympathomimetics: Concurrent use with other ADHD stimulants, weight-loss pills, or decongestants containing pseudoephedrine or phenylephrine can excessively raise heart rate and blood pressure.
- Serotonergic drugs: Selective serotonin reuptake inhibitors (SSRIs), SNRIs, certain migraine medications (triptans), tramadol, and St. John’s wort may increase the risk of serotonin syndrome when combined, especially at higher doses.
- Blood pressure or heart medicines: Some antihypertensives may be less effective, while other cardiac drugs can have more complex interactions; clinicians may need to adjust doses and monitor more closely.
- Acidifying and alkalinizing agents: Large amounts of vitamin C or acidic fruit juices can lower amphetamine levels and reduce effect, whereas antacids or medications that alkalinize the urine (such as sodium bicarbonate or certain diuretics) can raise levels and increase side effects.
- Other psychiatric medications: Tricyclic antidepressants, bupropion, and antipsychotics can interact pharmacodynamically (for example, increasing blood pressure or opposing stimulant effects), so combined use requires careful monitoring.
Conditions warranting extra caution or avoidance:
- Structural heart disease, cardiomyopathy, serious arrhythmias, coronary artery disease, or a history of stroke.
- Moderate to severe high blood pressure or advanced hardening of the arteries.
- Hyperthyroidism, glaucoma, or severe anxiety or agitation.
- History of substance use disorder, bipolar disorder, psychosis, or significant tics or Tourette syndrome.
- Seizure disorders or severe kidney impairment, which may require dose adjustments and closer observation.
Monitoring needs:
- Before starting therapy, clinicians typically review personal and family history for heart disease or sudden death and check blood pressure and heart rate.
- During treatment, blood pressure, heart rate, and weight (and height in children) are monitored regularly, along with mood, behavior, and signs of misuse or diversion.
- Additional tests, such as an electrocardiogram (ECG) or lab work, may be ordered if there are cardiac risk factors, symptoms, or other medical conditions.
Common Questions and Answers
Q: How quickly will dextroamphetamine sulfate start working for ADHD or narcolepsy?
A: Immediate-release forms usually begin to work within about 30–60 minutes of a dose, with peak effects in a few hours, so many people notice improved focus or wakefulness the first day at an effective dose, while overall benefit is fine-tuned over several weeks of dose adjustments.
Q: How is dextroamphetamine different from Adderall or methylphenidate?
A: Dextroamphetamine contains only the dextro isomer of amphetamine, whereas Adderall is a mixture of amphetamine salts and methylphenidate is a different stimulant class; in practice, their overall effectiveness is similar, but individuals may respond better or have fewer side effects with one product versus another.
Q: Can I drink alcohol while taking dextroamphetamine sulfate?
A: Alcohol can mask how impaired you feel, may worsen judgment and heart-related side effects, and can interfere with sleep, so most clinicians advise avoiding or minimizing alcohol and never using it to "come down" from the medication’s effects.
Q: Will this medication cause dependence or addiction?
A: Dextroamphetamine is a Schedule II controlled substance with potential for misuse and dependence, but when it is taken as prescribed and monitored, the risk is much lower; your prescriber will screen for substance use issues and may use pill counts or prescription monitoring to help ensure safe use.
Q: Does dextroamphetamine stunt growth in children?
A: Some children have slowed weight gain and, less often, slowed height growth while on stimulant treatment, so healthcare providers regularly track height and weight and may adjust the dose, timing, or schedule (including possible medication breaks) if growth appears to be affected.
Q: What should I do if I feel the medication has stopped working as well?
A: Do not increase the dose on your own; instead, talk with your prescriber, who can review adherence, timing, sleep and stress, other medications, and possible tolerance or progression of symptoms, and then adjust the dose, switch formulations, or consider alternative treatments if needed.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Store dextroamphetamine sulfate at room temperature (about 68–77°F / 20–25°C) in a tightly closed, child-resistant container, away from moisture, heat, and direct light, and keep it locked or otherwise secured because it is a Schedule II controlled substance.
Safety in the home: Keep out of sight and reach of children, teens, and visitors; count pills periodically and do not share this medicine with anyone, as misuse can cause serious harm and is illegal.
Disposal: When no longer needed, use a drug take-back program (pharmacies, clinics, or community events) if available; if none is accessible, follow local guidance, which often recommends mixing the medicine (without crushing extended-release beads) with an unappealing substance (such as used coffee grounds or cat litter), sealing in a bag or container, and placing it in household trash rather than flushing, unless the product label or local authorities specifically instruct otherwise.