At a Glance
How It Works
- Clonidine works mainly in the brain by stimulating alpha-2 adrenergic receptors, which reduce the "fight or flight" nerve signals that tighten blood vessels.
- This relaxes blood vessels and slows the heart slightly, helping lower blood pressure.
- In ADHD, the calming effect on nerve activity helps reduce hyperactivity, impulsivity, and improve attention.
Treatment and Efficacy
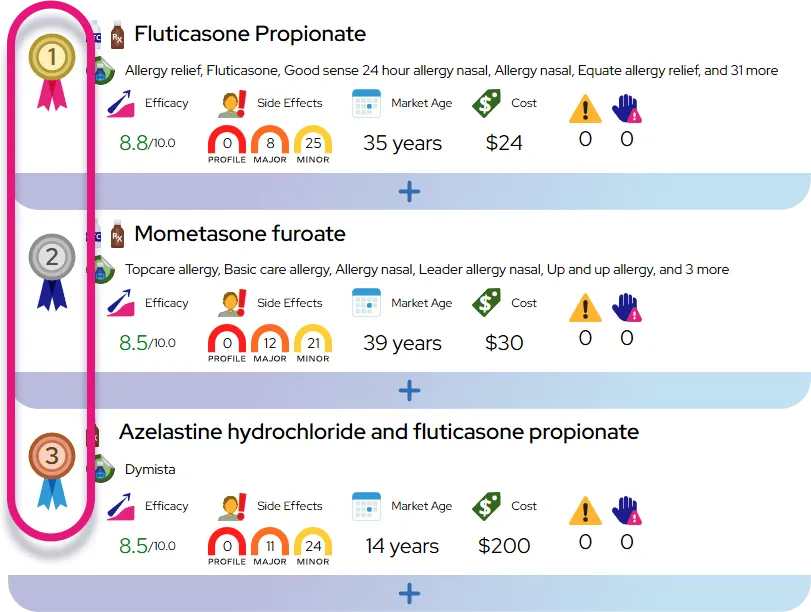
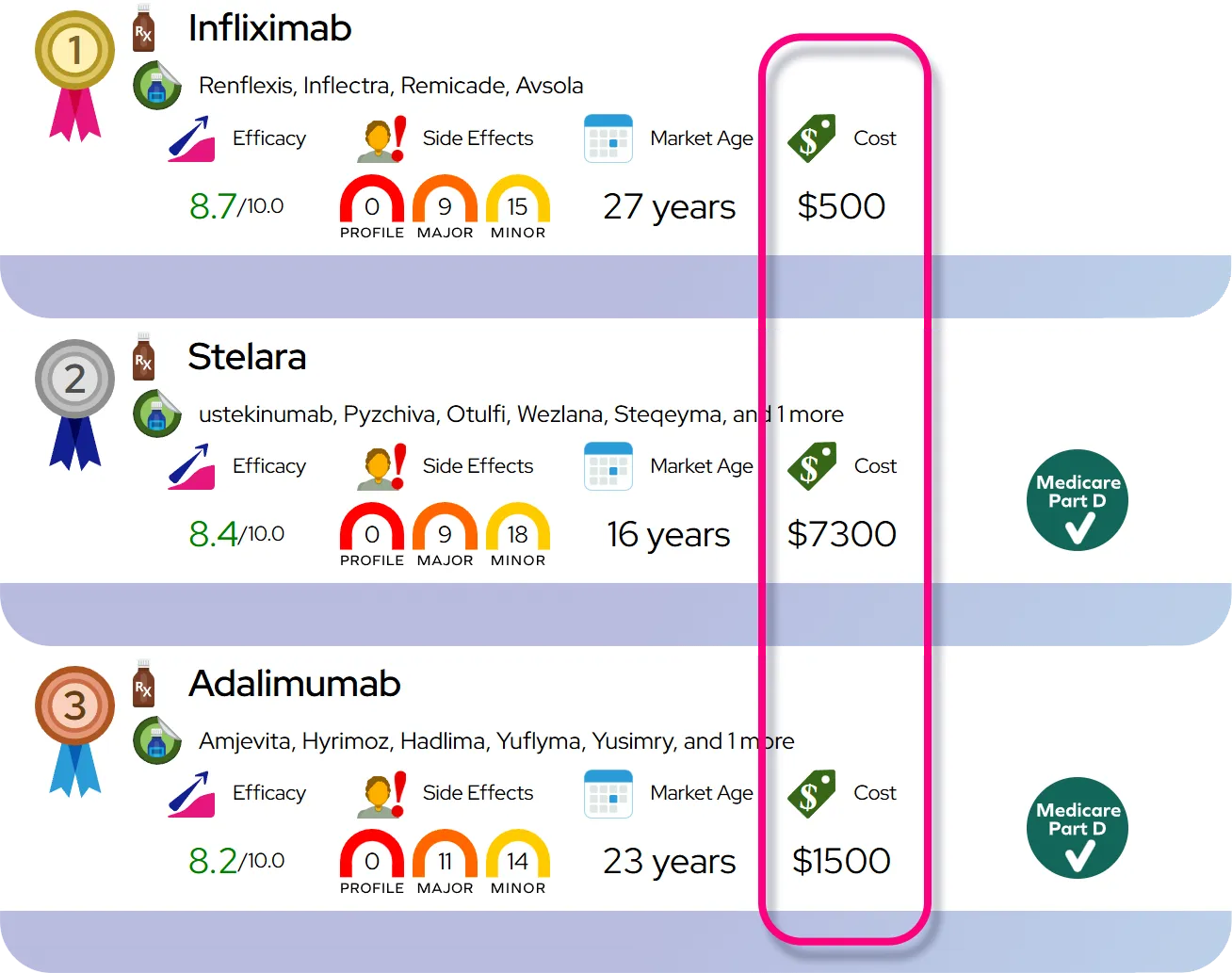
Approved indications: Oral clonidine is approved to treat hypertension in adults (alone or with other blood pressure medicines), and extended-release clonidine (e.g., Kapvay) is approved for ADHD in children and adolescents 6–17 years, usually as part of a total treatment program.
Off-label uses and evidence: Clinicians may prescribe oral clonidine off-label for conditions such as hot flashes/menopausal vasomotor symptoms, tic disorders (including Tourette syndrome), opioid or nicotine withdrawal symptoms, anxiety, sleep problems related to ADHD, and certain pain or autonomic conditions; supporting evidence ranges from moderate (e.g., menopausal hot flashes, tics, withdrawal symptoms) to limited or small studies for other uses.
Efficacy expectations in hypertension: Blood pressure often begins to fall within hours of a dose, with full effect over several days of regular use; it can substantially lower systolic and diastolic blood pressure, similar to other centrally acting agents, but is generally not a first-line medication compared with drugs like thiazide diuretics, ACE inhibitors, or ARBs.
Efficacy expectations in ADHD: For ADHD, effects on hyperactivity, impulsivity, and irritability may appear within 1–2 weeks and continue to improve over several weeks; it is usually somewhat less potent for core attention symptoms than stimulant medications but can be useful when stimulants are not tolerated, are insufficient alone, or when sleep problems, aggression, or tics are also present.
Dosage and Administration
Typical adult dosing for hypertension (immediate-release): Often starts at about 0.1 mg twice daily, with gradual increases (usually by 0.1 mg/day at weekly intervals) based on blood pressure response; total daily doses commonly range from 0.2–0.6 mg in divided doses, and should not be adjusted without clinician guidance.
Typical pediatric dosing for ADHD (extended-release): Extended-release tablets are usually started at 0.1 mg at bedtime, then titrated by 0.1 mg/day at weekly intervals up to a usual total daily dose of 0.2–0.4 mg, taken once daily at bedtime or split twice daily, depending on the specific product instructions.
How to take it: Swallow tablets whole with water; extended-release tablets should not be crushed, chewed, or broken. Clonidine can be taken with or without food but should be taken consistently the same way each day. Because it can cause drowsiness, many people take at least one dose at bedtime.
Special dosing instructions: Do not stop clonidine suddenly; doses should be tapered slowly under medical supervision to avoid rebound high blood pressure and other withdrawal symptoms. If another blood pressure or ADHD medicine is added or removed, your clinician may adjust your clonidine dose.
Missed dose guidance: If you miss a dose and it is only been a short time, take it when you remember; if it is almost time for your next dose, skip the missed dose and resume your regular schedule—do not double up doses, and contact your prescriber if you miss more than one dose, especially with high blood pressure.
Overdose: Taking too much clonidine can cause severe drowsiness, very low blood pressure, slow heart rate, shallow breathing, or fainting; this is an emergency, and immediate medical attention or poison control center contact is needed.
Safety and Side Effects
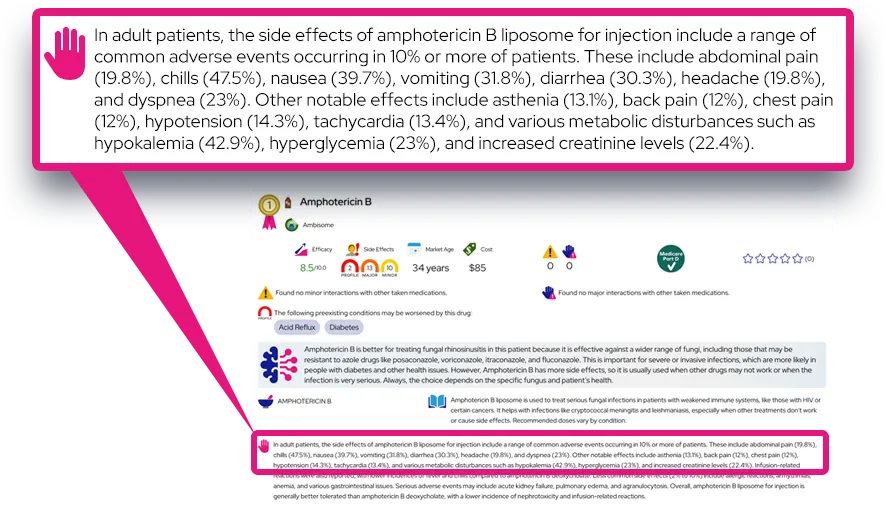
Common side effects: Frequently reported effects include drowsiness, fatigue, dry mouth, dizziness or lightheadedness (especially when standing), constipation, and headache; these often start soon after beginning treatment or dose increases and may lessen over time, but can remain bothersome in some people.
Serious or rare adverse effects: Serious problems can include very low blood pressure, slow heart rate (bradycardia), fainting, severe dizziness, chest pain, shortness of breath, confusion, or signs of an allergic reaction such as rash, swelling of the face or throat, or trouble breathing; abrupt stopping can cause rebound high blood pressure, rapid heart rate, agitation, or headache and needs urgent care.
Warnings and precautions: Use with caution in people with heart conduction problems, severe coronary artery disease, recent heart attack, stroke, kidney disease, or those prone to low blood pressure; dose adjustments may be needed in kidney impairment. In pregnancy, clonidine is sometimes used when benefits outweigh risks, but other blood pressure medicines are often preferred; it passes into breast milk, so breastfeeding decisions should be individualized with a clinician. Extended-release clonidine for ADHD is approved for ages 6–17; in very young children, use is off-label and must be closely supervised.
Relative safety compared with other drugs: Compared with many first-line blood pressure medicines, clonidine causes more sedation, dry mouth, and risk of rebound hypertension if doses are missed or the drug is stopped suddenly, so it is often reserved for specific situations or when other options are inadequate.
Side-effect reporting and safety updates: Patients in the United States can report side effects to the FDA through the MedWatch program (online or by phone), and up-to-date safety communications are available on the FDA’s website or through their healthcare provider or pharmacist.
Interactions and Precautions
Drug interactions: Clonidine can enhance the sedative or blood pressure–lowering effects of other medicines, including benzodiazepines, opioids, many sleep aids, other blood pressure medicines, and some antipsychotics; combining with beta-blockers requires careful management, especially when changing doses, to avoid dangerous rebound hypertension or heart problems.
OTC medicines, supplements, and alcohol: Over-the-counter products that lower blood pressure or cause drowsiness (such as certain cold medicines, antihistamines, or sleep aids) can add to clonidine’s effects. Alcohol increases sedation and dizziness and can further lower blood pressure, so it is best limited or avoided.
Food and procedures: There are no major food restrictions with clonidine, but standing up slowly and staying hydrated can help reduce dizziness. Clonidine should usually be continued before most imaging or surgical procedures, unless the medical team instructs otherwise, to prevent rebound high blood pressure from sudden withdrawal.
Precautions and when use may be unsafe: Extra caution is needed in people with low baseline blood pressure, serious heart rhythm or conduction problems, severe coronary artery disease, recent heart attack or stroke, kidney impairment, depression, or a history of fainting; dose adjustments or alternative therapies may be preferred in these situations.
Monitoring needs: Regular monitoring of blood pressure and heart rate is important, especially when starting, changing doses, or adding other blood pressure or heart medicines; some patients, particularly those with heart disease, may require additional heart monitoring (such as ECGs) based on their clinician’s judgment.
Common Questions and Answers
Q: How long does it take oral clonidine to start lowering blood pressure?
A: Blood pressure often begins to decrease within a few hours of a dose, with a more stable effect after several days of regular use.
Q: Can clonidine make me sleepy or affect my concentration?
A: Yes, drowsiness, fatigue, and slowed thinking can occur, especially when starting or increasing the dose, so caution with driving or operating machinery is advised until you know how it affects you.
Q: What happens if I suddenly stop taking clonidine?
A: Stopping clonidine abruptly can cause rebound high blood pressure, rapid heart rate, headache, and nervousness, so it should be tapered gradually under medical supervision.
Q: Is clonidine addictive?
A: Clonidine is not considered addictive in the way many controlled substances are, but your body does become used to it, so sudden discontinuation can cause withdrawal-like symptoms and must be avoided.
Q: Can I take clonidine with my other blood pressure or ADHD medications?
A: Clonidine is often used together with other blood pressure or ADHD medicines, but combinations must be carefully managed by your prescriber to avoid excessive lowering of blood pressure, heart rate changes, or added side effects.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Store clonidine tablets at room temperature (generally 68°F–77°F or 20°C–25°C), away from excess heat, moisture, and direct light, and keep the bottle tightly closed.
Child safety: Keep out of reach of children and pets, as even a few tablets can cause dangerous drops in blood pressure and heart rate.
Disposal: Use a community drug take-back program when possible; if unavailable, mix unused tablets with an unappealing substance (such as used coffee grounds or kitty litter), place the mixture in a sealed container, and throw it in the household trash, and remove or black out personal information on prescription labels.