At a Glance
How It Works
- Atomoxetine is a non-stimulant medicine that works mainly by blocking the reuptake of norepinephrine (a brain chemical), so more of it stays available between nerve cells.
- This helps brain circuits involved in attention, impulse control, and organization work more smoothly over the whole day.
- Because it does not increase dopamine in reward pathways like stimulants, it has very low abuse potential and is not a controlled substance.
Treatment and Efficacy
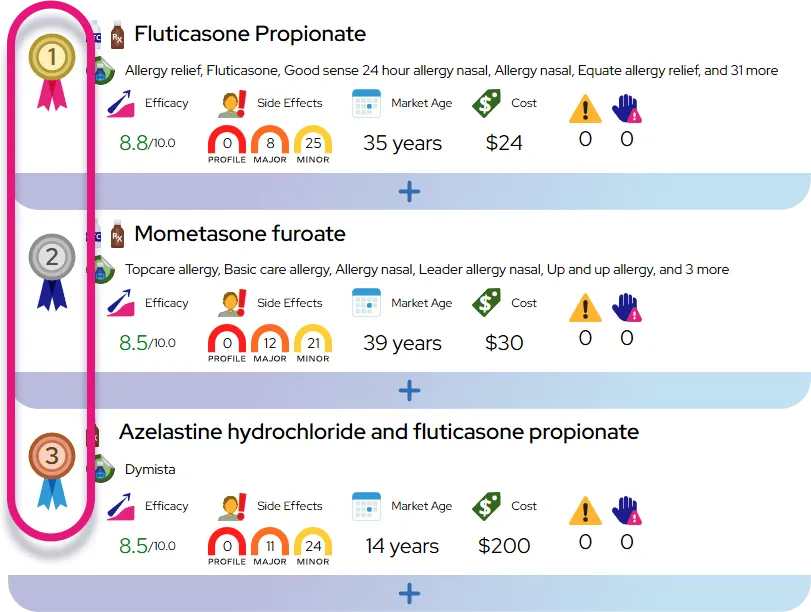
Approved indications: Atomoxetine is approved in the United States for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children, adolescents, and adults aged 6 years and older, regardless of ADHD subtype.
Common off-label uses: Clinicians may use atomoxetine off-label for ADHD in patients who cannot take stimulants (for example, those with tic disorders, anxiety disorders, or substance use risk) and sometimes as an adjunct to stimulants when a full-day effect is needed; evidence for these uses is moderate, coming from clinical trials and observational studies but not formal FDA approval.
Efficacy expectations and onset: Many patients notice small improvements in attention, impulsivity, or hyperactivity within 1–2 weeks, but clinically meaningful benefit often takes 4–6 weeks at a stable dose, and some patients continue to improve over 3–6 months.
Typical clinical outcomes: On average, about half of patients reach a clearly meaningful reduction in core ADHD symptoms, with better focus, less impulsive behavior, and improved organization; others may experience partial benefit or mainly improvement in specific domains such as emotional control or late-day symptoms.
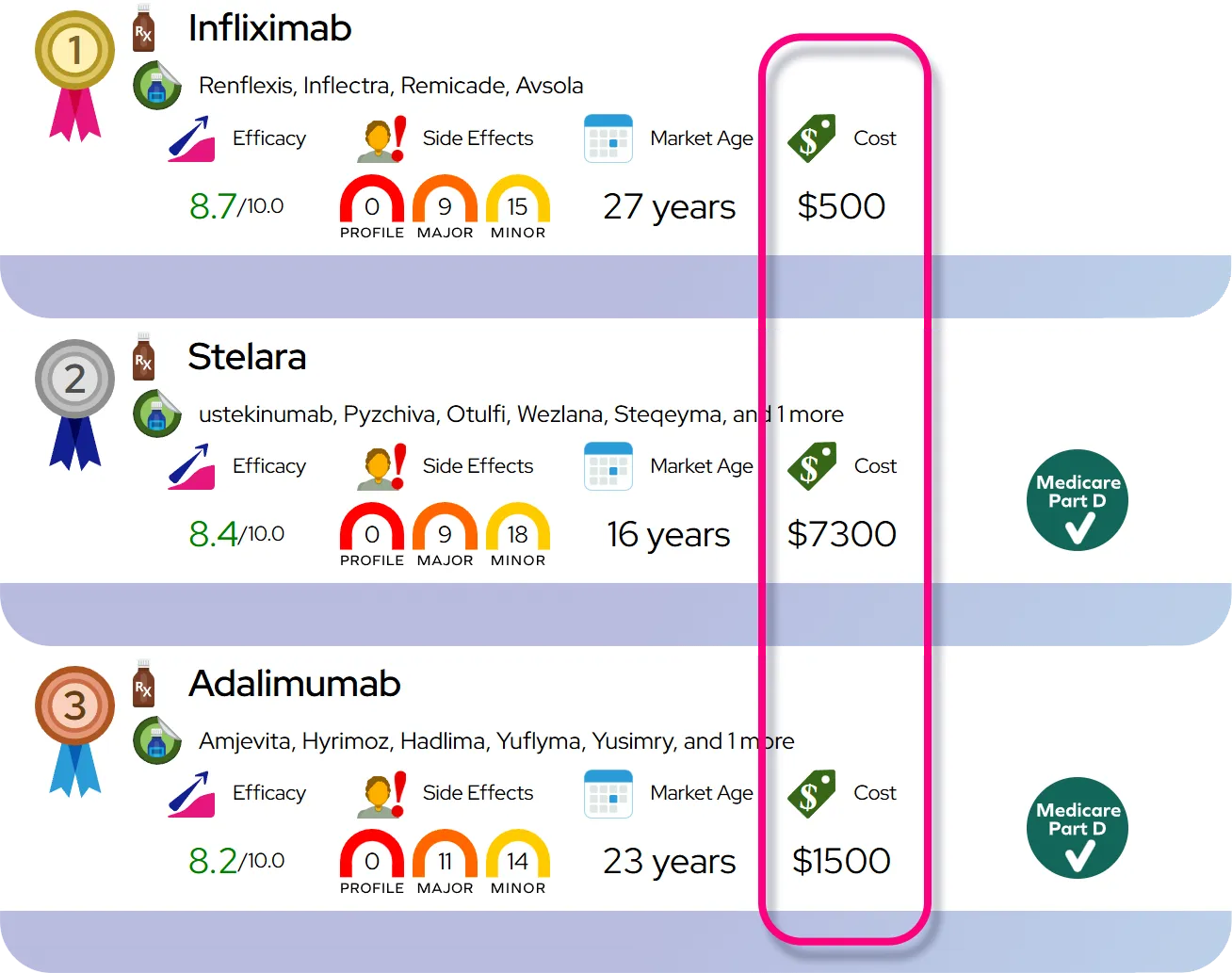
Comparison with stimulants: Stimulant medications (such as methylphenidate and amphetamine products) usually work faster and, for many people, reduce symptoms somewhat more strongly, but atomoxetine offers 24-hour coverage, has very low abuse potential, and can be preferable when stimulant side effects, contraindications, or misuse concerns are present.
Dosage and Administration
Usual adult dosing (and children/adolescents over 70 kg): Start with 40 mg by mouth once daily; after at least 3 days, increase to a target of about 80 mg/day, given once in the morning or split into morning and late-afternoon/early-evening doses; if needed, the dose may be raised after another 2–4 weeks up to a maximum of 100 mg/day.
Usual pediatric dosing (children and adolescents 6 years and older, 70 kg or less): Start at 0.5 mg/kg/day; after at least 3 days, increase to a target of about 1.2 mg/kg/day once daily in the morning or in two divided doses; the total daily dose should not exceed 1.4 mg/kg/day or 100 mg/day, whichever is lower.
How to take the medicine: Swallow capsules whole with water; do not open, crush, or chew them. Atomoxetine may be taken with or without food. It is commonly taken once daily in the morning, but some patients do better with the dose split between morning and late afternoon/early evening.
Special dosing considerations: In moderate liver impairment, both the starting and target doses are usually reduced to about half; in severe liver impairment, to about one quarter of the usual dose. When used with strong CYP2D6 inhibitors (such as fluoxetine, paroxetine, or quinidine), doses are usually started low (for example, 0.5 mg/kg/day in smaller patients or 40 mg/day in larger patients) and increased more slowly, only if symptoms do not improve after several weeks and the lower dose is well tolerated.
Missed dose guidance: If you miss a dose, take it as soon as you remember the same day, but if it is close to the time for the next dose, skip the missed dose and resume your regular schedule; do not take more than the prescribed total daily amount in any 24-hour period.
Overdose: In case of overdose or accidental ingestion (especially in a child), call poison control (1-800-222-1222 in the U.S.) or seek emergency medical care immediately; symptoms may include severe drowsiness, agitation, fast heartbeat, high blood pressure, stomach upset, or seizures.
Safety and Side Effects
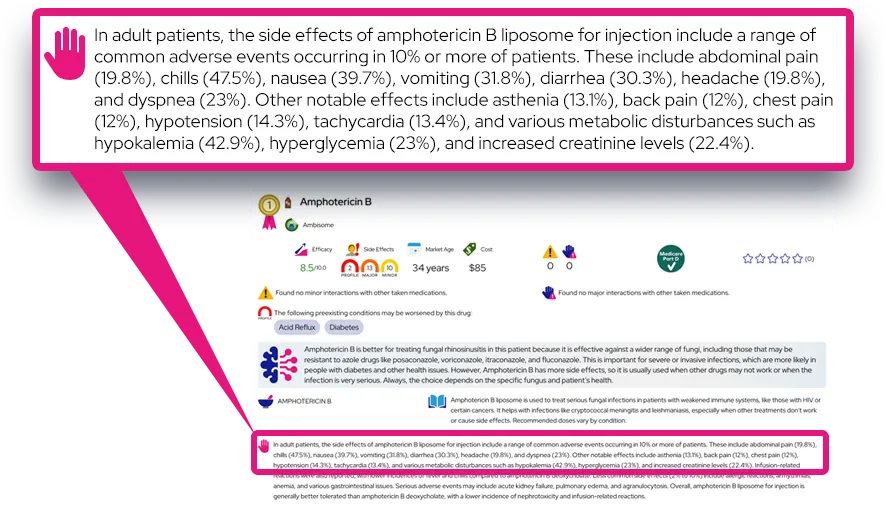
Common side effects (often mild to moderate):
- Decreased appetite, nausea, stomach pain, vomiting, dry mouth, or constipation, especially in the first days to weeks.
- Trouble sleeping or, less often, sleepiness; headache, dizziness, or fatigue.
- Slight increases in heart rate and blood pressure, usually small but sometimes noticeable.
- In adults, sexual side effects such as decreased libido or difficulty with erection or orgasm.
These effects are fairly common, tend to appear early in treatment or after dose increases, and often lessen with time; dose adjustment, taking the dose earlier in the day, or taking it with food may help.
Serious or rare adverse effects (seek immediate medical attention):
- New or worsening suicidal thoughts, self-harm behavior, severe agitation, or unusual behavioral changes, especially in children and adolescents during the first few months or after dose changes.
- Signs of severe liver injury: dark urine, yellowing of skin or eyes, severe fatigue, right upper abdominal pain, flu-like symptoms—if suspected, the drug should be stopped and not restarted if liver injury is confirmed.
- Serious cardiovascular events in susceptible people: chest pain, fainting, shortness of breath, fast or irregular heartbeat, or stroke-like symptoms (sudden weakness, trouble speaking, vision changes).
- Allergic reactions such as rash, hives, swelling of the face, lips, tongue, or throat, or trouble breathing.
- Urinary retention or very painful/prolonged erections (priapism) in males.
Warnings and precautions:
- Mood and behavior: Monitor all patients—especially children, teens, and young adults—for suicidal thinking, depression, irritability, or unusual behavior changes.
- Heart and blood vessels: Use caution and perform careful evaluation in people with serious heart disease, structural heart defects, cardiomyopathy, arrhythmias, moderate-to-severe hypertension, or history of stroke.
- Liver disease: Dose reductions are usually needed in moderate or severe hepatic impairment, and treatment should be stopped if clear liver injury occurs.
- Glaucoma and pheochromocytoma: Atomoxetine is contraindicated in narrow-angle glaucoma and in patients with pheochromocytoma or a history of pheochromocytoma.
- MAOIs: Do not use with monoamine oxidase inhibitors (MAOIs) or within 14 days of MAOI use, because of the risk of serious reactions.
- Pregnancy and breastfeeding: Human data are limited; use only if the expected benefit justifies potential risks, and discuss breastfeeding decisions with the prescriber.
- Age limits: Safety and effectiveness have not been established in children younger than 6 years.
Relative safety compared with other ADHD drugs: Atomoxetine is not a controlled substance and has very low abuse and dependence potential; it tends to cause more gastrointestinal and fatigue-type side effects than some stimulants but avoids stimulant-related issues such as misuse and marked appetite or sleep disruption in many patients.
Reporting side effects and staying updated: Patients and caregivers should report troublesome or serious side effects to the prescriber and can report directly to the FDA MedWatch program (online or by phone); updated safety information and Medication Guides are available from the FDA and the pharmacy dispensing the medicine.
Interactions and Precautions
Major drug interactions:
- MAOIs: Atomoxetine must not be used with monoamine oxidase inhibitors or within 14 days of stopping an MAOI, due to risk of serious, sometimes life-threatening reactions.
- CYP2D6 inhibitors: Medicines that strongly inhibit CYP2D6—such as fluoxetine, paroxetine, quinidine, and some others—can raise atomoxetine levels, increasing side effects; lower starting doses and slower titration are recommended.
- Other drugs that raise blood pressure or heart rate: Decongestants (like pseudoephedrine), stimulant ADHD medications, and some asthma drugs can add to atomoxetine’s cardiovascular effects, so blood pressure and pulse should be monitored.
- Drugs affecting heart rhythm or seizures: Use caution with medicines that prolong the QT interval or lower the seizure threshold, as atomoxetine can modestly affect heart rate and, rarely, seizures.
Alcohol, food, and supplements: There are no major food interactions, and atomoxetine can be taken with or without food; alcohol does not strongly interact pharmacokinetically but may worsen side effects such as dizziness or judgment impairment, so limiting alcohol is advised. Herbal products or supplements that stimulate the nervous system or raise blood pressure (for example, high-dose caffeine or some weight-loss products) should be used cautiously.
Conditions that require extra caution or may make use unsafe:
- Serious structural heart disease, cardiomyopathy, significant arrhythmias, or uncontrolled hypertension.
- Narrow-angle glaucoma, pheochromocytoma or history of pheochromocytoma.
- Moderate to severe liver disease or a prior episode of atomoxetine-related liver injury.
- History of bipolar disorder, psychosis, or seizures, where careful monitoring for mood or neurologic changes is needed.
Monitoring needs: Before and during treatment, clinicians typically check blood pressure and heart rate, assess for cardiac risk factors, and monitor mood, behavior, and suicidal thoughts, especially in children, adolescents, and young adults. In children and teens, growth (height and weight) should be followed over time. Liver function tests may be obtained if there are symptoms suggesting liver injury.
Common Questions and Answers
Q: How long does it take for atomoxetine to start working for ADHD?
A: Some people notice small improvements within 1–2 weeks, but it usually takes 4–6 weeks at a stable dose to see clear benefit, and in some patients improvements continue to build over 3–6 months.
Q: Is atomoxetine a stimulant or a controlled substance?
A: Atomoxetine is a non-stimulant selective norepinephrine reuptake inhibitor and, unlike stimulant ADHD medicines, it is not classified as a controlled substance and has very low abuse potential.
Q: Can I stop atomoxetine suddenly?
A: Atomoxetine does not typically cause dependence, so it can usually be stopped without tapering, but symptoms of ADHD may gradually return, so any changes should be planned with your prescriber.
Q: What should I do if I feel very nauseated or lose my appetite on atomoxetine?
A: These side effects are common early in treatment and often improve over time; taking the medicine with food, adjusting the timing, or changing the dose can help, so discuss persistent or severe symptoms with your clinician.
Q: Can atomoxetine affect my sleep?
A: Atomoxetine can cause either trouble falling asleep or, less often, sleepiness; taking the dose earlier in the day or splitting it into morning and late-afternoon doses may reduce sleep problems.
Q: Is it safe to drink coffee or other caffeinated drinks while taking atomoxetine?
A: Moderate caffeine is usually acceptable, but because both caffeine and atomoxetine can increase heart rate or cause jitteriness in some people, it is sensible to limit very high caffeine intake and monitor how you feel.
Better Treatment, Lower Cost – No Catch.
Find safer, more effective medications with fewer side effects – often for less money. It’s fast, free, and personalized. Learn More →
Disposal Guidance
Storage: Store atomoxetine capsules at room temperature (about 68–77°F / 20–25°C), tightly closed in the original container, away from moisture, heat, and direct light, and out of reach of children and pets.
Handling: Swallow capsules whole and do not open them; if a capsule breaks, avoid touching the powder with bare hands and wash the area if contact occurs.
Disposal: If you no longer need the medication or it is expired, use a community drug take-back program if available; if not, mix the capsules (kept closed) with an undesirable substance (such as used coffee grounds or kitty litter), place the mixture in a sealed bag or container, and throw it in the household trash—do not flush atomoxetine down the toilet unless specifically instructed.